PART 3. WHAT IF THEY EXISTED? CHEMICAL AND PHYSIOLOGICAL CONSEQUENCES
POINTS TO CONSIDER


Part 2 of the topic explained how all the evidence used to support microclustering is invalid. Here are a couple common sense ideas that refute the microclustering myth without all the technical science.
COLLIGATIVE PROPERTIES ARE THE SAME
If there really were a difference in the water’s structure; that is, if there were any microclusters, then the hydrogen bond network would obviously be different (because the hydrogen bond network is what would form the clusters).1,2 Therefore, if the hydrogen bond network were different, then this would absolutely have to alter the colligative properties of water.3 For example, differences in boiling and freezing points, specific gravity, etc.4 Even adding solutes (ions, minerals, etc.) to the water alters these properties.5 This is known as “molal freezing point depression”6 and “molal boiling point elevation”.7 That is why we add salt to the roads and sidewalks in the winter because it lowers the freezing point of the water.8
However, studies9—including my own—have found no difference between ionized water and other water with the same osmolarity (number of solutes per liter aka minerals, ions, etc.)
NONE OF THE IONIZED WATER SCIENTISTS CLAIM MICROCLUSTERING
Another important observation is that none of the scientists that are publishing articles on ionized water make claims regarding microclustering.10 While in Japan, I discussed water microclusters with a number of top researchers. They all emphatically denied its existence and called it pseudoscience. This begs the question as to why marketers of this water are claiming the very thing that their supporting scientists are denying?
A MICROCLUSTER IS ACTUALLY REALLY LARGE
The figure below demonstrates the size of a “microcluster” with respect to normal water and cells.
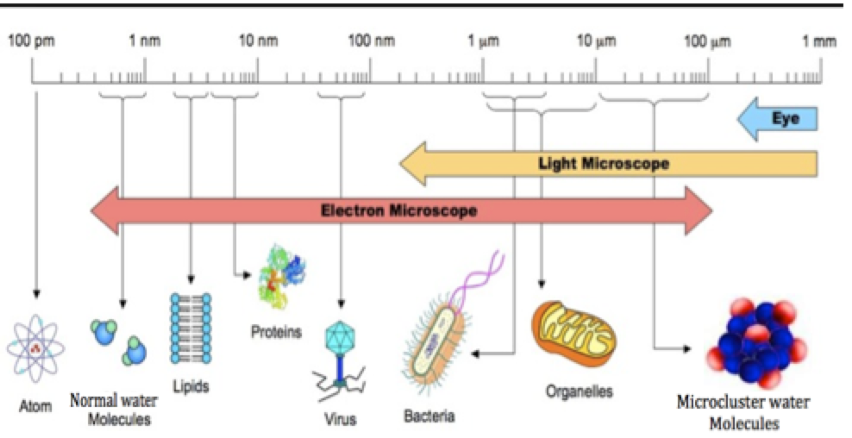

Ironically, if electrolysis really turned normal water into microclusterd water, the water molecule would be about as big as the cell itself.11 A cell is only around 10-100 micrometers long,11 and a water molecule is less than a nano-meter,12 which is a thousand times smaller than a micrometer.


The idea of “micro-clustering” is akin to me saying that ionized water has ‘cellular vibrational frequencies’ (note: I just made up that term) that ‘heal your body’. Even though there is no scientific evidence for it, I’ll just claim that science just hasn’t developed instrumentation sensitive enough to detect its presence. I am sure the fallacy in such logic is clear; unfortunately, it’s all too common.13
NO PHYSICAL OR CHEMICAL EVIDENCE…EXCEPT TO REFUTE IT
In short, it is very clear that stable microclusters of any shape have never been observed.9 This is extremely clear from a recent high-quality article, which used multi-dimensional nonlinear infrared spectroscopy to analyze water dynamics. It was found that the redistribution of the hydrogen bond network of liquid H2O was around 50 femtoseconds!14 That is less than 1/10 millionth of a millionth of a second, which is more than a magnitude faster than any other liquid.15 Indeed there are no stable clusters of any size in liquid water.16 And as discussed previously there has been a lot of research into water including Monte Carlo simulations17 and ab initio computational analysis.18
So far, from a physical and chemical viewpoint, there has been no valid evidence to support the existence of stable microclusters,16 and research in this field continuously refutes the claim,9 which comes as no surprise for the claim contradicts basic and fundamental principles of chemistry.19 Therefore, it is absolutely impossible to claim that ionized water is “micro-clustered” and tap water is not. Or that only machine A can produce microclusters, or this machine produces more (or smaller) microclusters than another machine. Or a higher wattage produce more (and smaller) microclusters? Couldn’t the same logic be used to say that a higher wattage actually produces less (and larger) microclusters? Such declarations are absurd and illogical. How can one compare imaginary (or at least immeasurable) properties?
SCIENCE AND CLAIMS
If science has not proven something false, does that make it true? For example, there is no scientific study that has disproven the idea that you will grow taller if you stand on your head and drink ionized water. So does that make the statement true?
Remember it is those who make the claims that bear the responsibility to provide evidence for those claims. If this were not so, then hucksters of any product could fabricate any claim and wait for the scientists to prove them wrong, (then move to another claim) and the more immeasurable and complex the claim the more successful their marketing campaign.
WHAT WOULD BE THE PHYSIOLOGICAL EFFECTS OF MICROCLUSTERING?
Because the existence of microclusters has no valid scientific evidence, their ascribed health effects are pointless and otiose. However, even if they did exist, would they really provide “numerous health benefits”?
MICROCLUSTERING & HYDRATION
The main purported reason that microclusters are a good thing is that they increase cellular hydration more than normal water.20 The logic is that large water clusters found in normal water can’t get into the cells, but small ones can easily penetrate them. A commonly used example is that of trying to throw tennis balls through a chain-link fence (normal water into cells) versus throwing pebbles through the fence (micro-clustered water into cells).21
This may sound great on the surface, but water rarely diffuses through cell membranes as it is—and that is a good thing. If it were able to penetrate the cells that easily, then we would not be able to retain water after filtration by the kidney.22 You would have to drink close to 180 liters of water per day, at a constant intake of 125 mL per minute23—not to mention how frequently you would have to empty your bladder!
AQUAPORINS
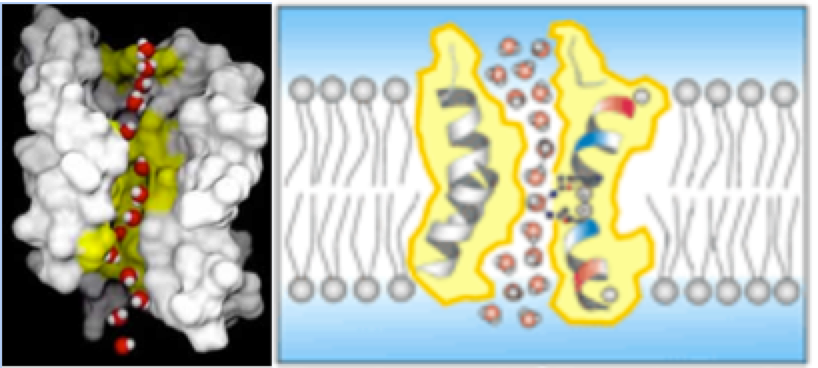

As seen in the above figure, water goes in and out of cells via aquaporins,24 and does so in a single file, separate, and isolated fashion.25 Not as clusters (see figure). The whole reason why scientists even thought that there must be water channels was because water could enter cells much faster than the rate of simple diffusion.26 Therefore, the only way this could be true would be to have water channels, which—thanks to Peter Agre—we have discovered.27
Ironically and quite comically, many microcluster proponents state that the Nobel Prize-winning aquaporin research somehow validates the importance of microclusters.28 Their explanations29,30 for the connection are highly inaccurate and complete pseudoscience. The fact that water travels through aquaporins is the epitome of a reason why microclustering would not matter because water enters the cell not as a cluster, but as a single molecule. In fact, if they were a stable microcluster then in order for it to be stable, the hydrogen bonds would have to be stronger, which would prevent the water from entering the cells.
If cells need to vary the rate of water uptake, they simply produce more or less aquaporins31—all of this is highly and specifically regulated.32
Hydration is determined by osmolarity33 and aquaporin activity.34 Remember osmosis? Now, I am well aware of the microcluster hydration studies using bioimpedance analyzers that claimed improved hydration.35 In fact, my first article on ionized water36 cited those studies. Unfortunately, they are simply not valid. They also contradict basic physiological and biological principles.37
Read MICROCLUSTERING: The Making Of A Myth (Part 4. Potential Negative Health Effects & Conclusions)







