What is Hydrogen?
Hydrogen gas has been shown to have a therapeutic effect on over 150 human diseases,1 but what is Hydrogen? Hydrogen is the lightest and simplest element with the symbol H. It consists of only one electron and one proton and, under normal conditions, it exists primarily in its diatomic form as molecular hydrogen (H2 gas). It is what powers the sun by fusion to produce helium. Hydrogen is the center of the prevailing cosmological model that describes the early development of the Universe2 as well as the origin of life itself.
FORMS OF HYDROGEN
ATOMIC HYDROGEN
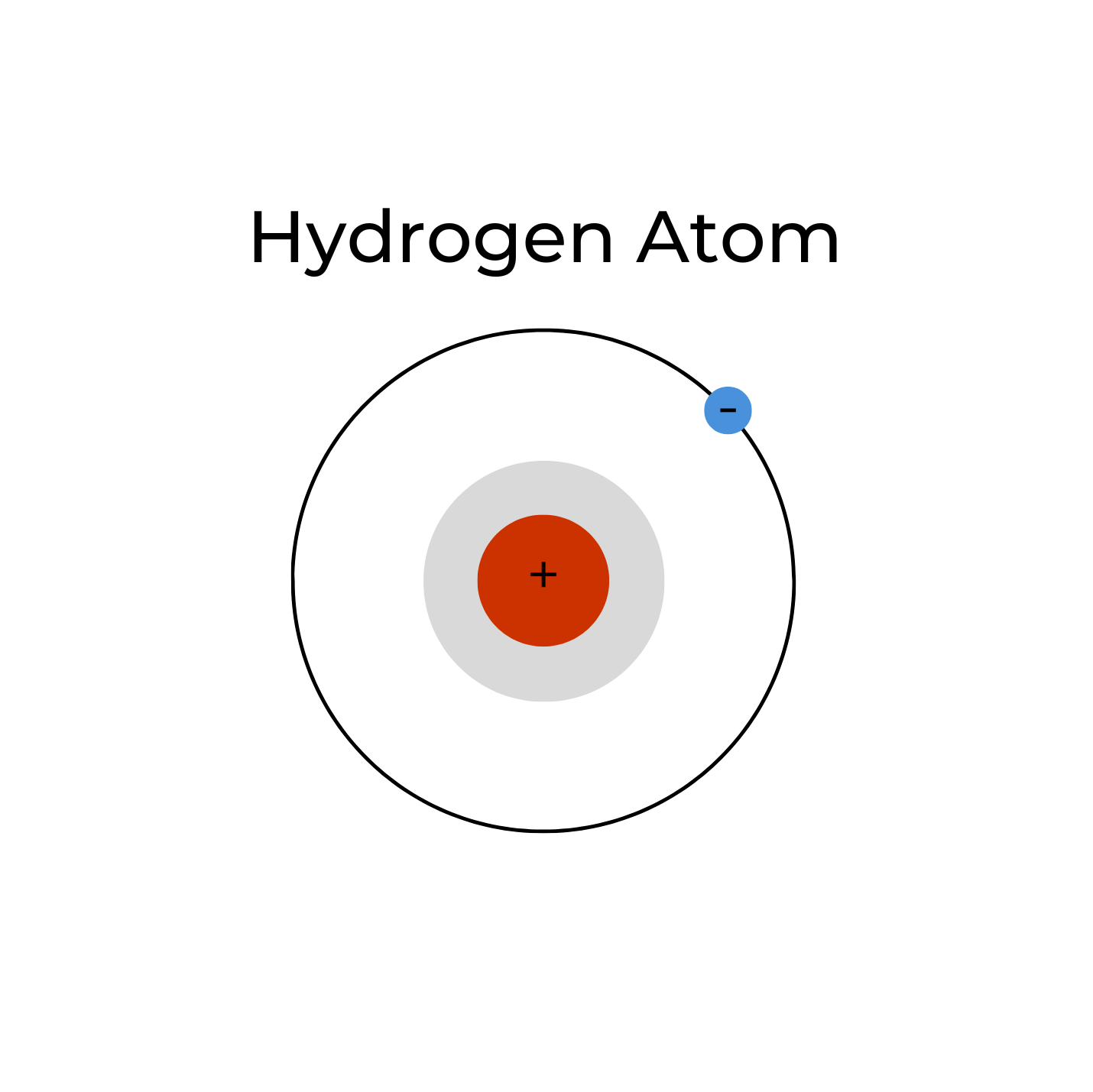

A single hydrogen atom (H•) is neutral and has an unpaired electron (as represented by the small dot “•”). It is, thus, a reactive free radical, which is why atomic hydrogen is rare. When atomic hydrogen is produced via electrolysis, it readily reacts with another hydrogen atom to form stable molecular hydrogen (H•+ H• => H2). In the 1990’s it was suggested that atomic hydrogen was responsible for the therapeutic effects of ERW3. This reactive atomic hydrogen was likely mistranslated from Japanese to English as “active hydrogen”3; however, the term “active hydrogen” is not a scientific term, and is frequently used in pseudo-scientific marketing hype. Moreover, the stable existence of atomic hydrogen in aqueous solutions remains unproven,4 and from a physical chemistry viewpoint, impossible. Atomic hydrogen was the first element in existence and is the first element on the Periodic Table; it can be considered the father of all the elements.
MOLECULAR HYDROGEN
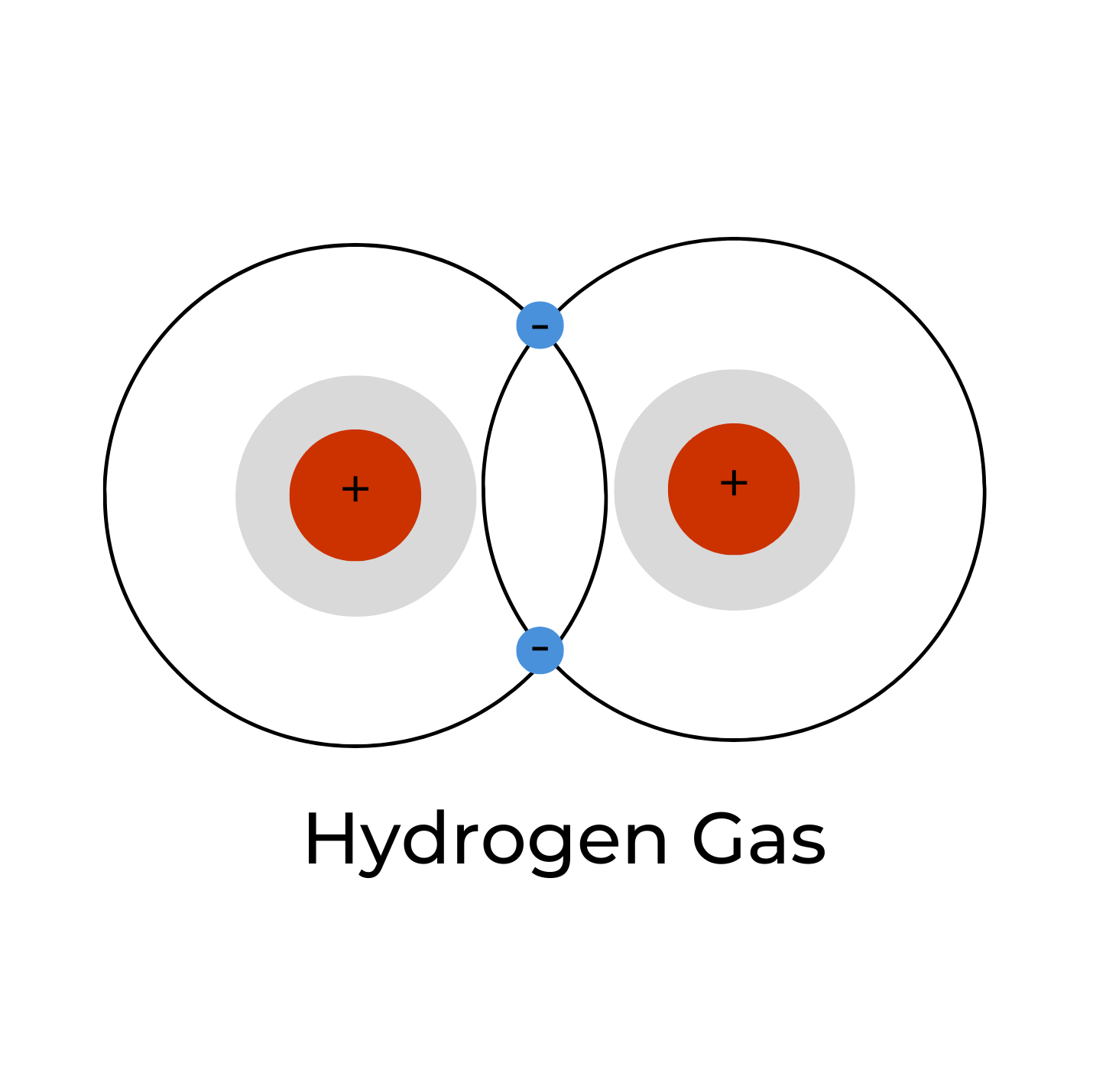

Molecular hydrogen gas, or H2 (g), is the primary form in which hydrogen is found. In other words, two hydrogen atoms (H) are covalently bonded (a type of chemical bond) together as H-H. Because there are two hydrogen atoms, we call this diatomic hydrogen (“di” meaning two). Because the hydrogen atoms are covalently bonded together, they form a molecule; so, H2 is also referred to as molecular hydrogen. We can also refer to it as dihydrogen. The hydrogen molecule contains two protons and two electrons making it a neutrally-charged molecule. It is a colorless, odorless, tasteless, non-metallic, highly flammable gas, and very explosive above a 4.6% concentration by volume. It is this form of hydrogen that has been shown to exert the wide range of therapeutic effects. Some articles refer to this as “hydrogen-rich water”. It is the smallest molecule in the universe, and this extremely small size and its high lipid solubility allows it to easily diffuse into the subcellular compartments of the mitochondria and other locations.5
HYDRIDE: THE NEGATIVE HYDROGEN ANION
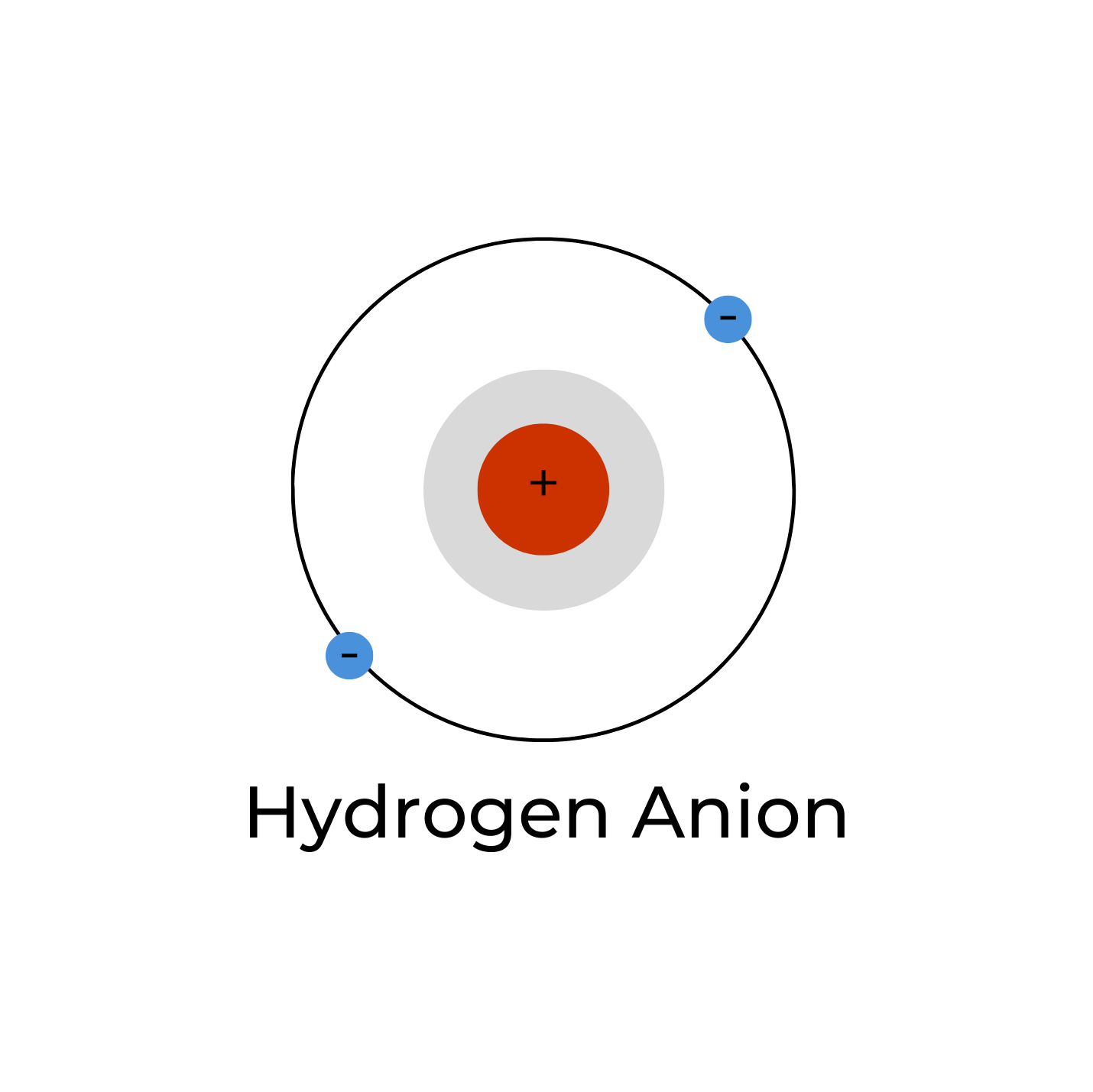

Hydride is one hydrogen atom that has an extra electron. It has one proton and two electrons, which makes it a negative ion written as H–. Because the hydrogen atom received an additional electron, it does not have an unpaired electron and thus is no longer a free radical. However, it is still not stable because, in this form, it is a very strong base. As such, it will react with water to produce molecular hydrogen (H– + H2O => H2 + OH–. 6 Most hydrides are chemical compounds (e.g. sodium borohydride, lithium aluminum hydride, etc.) used as reducing agents in chemical synthesis.
POSITIVELY-CHARGED HYDROGEN CATION: THE ‘H’ IN pH
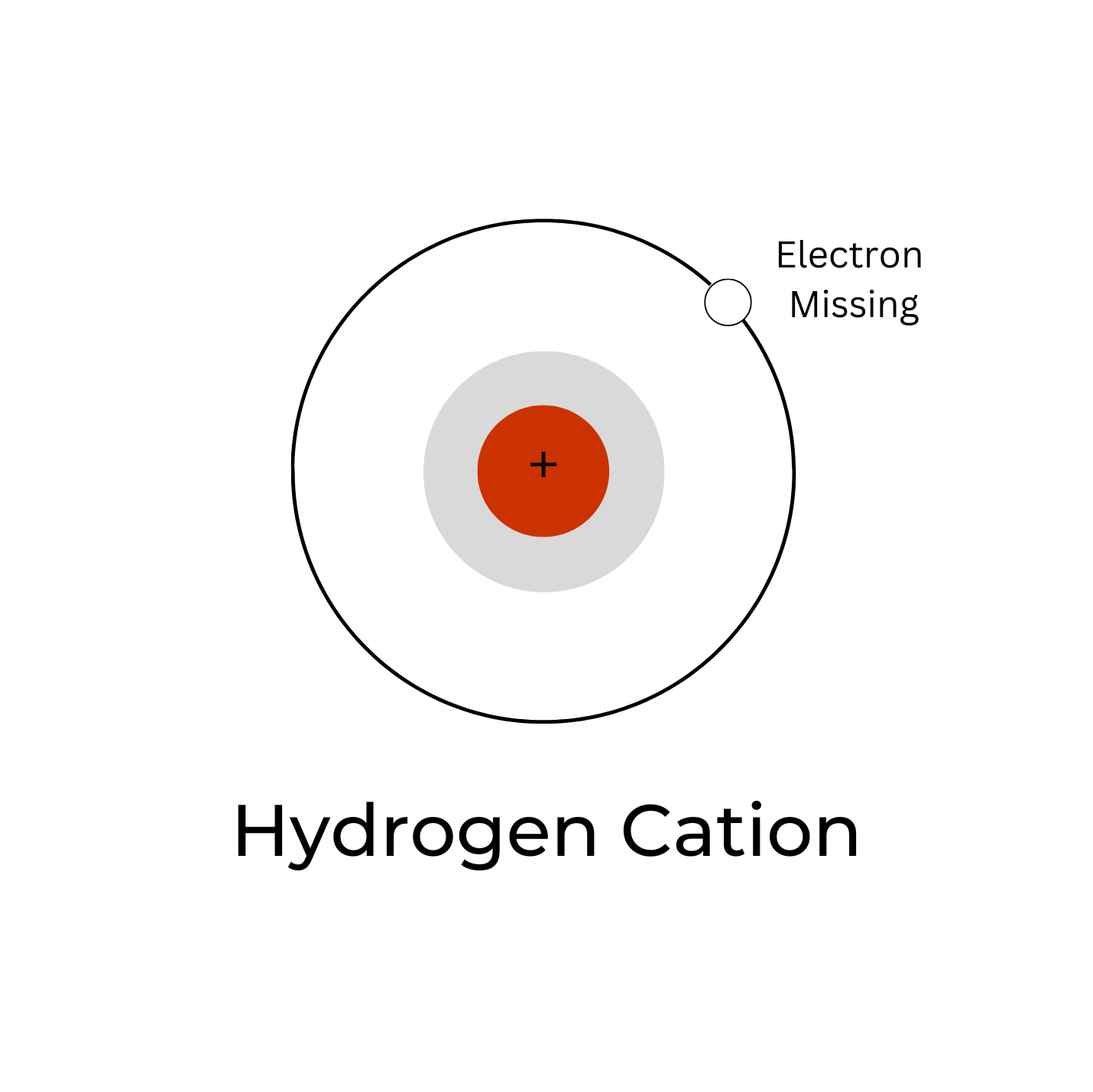

A positively charged hydrogen ion (H+ cation) is also known as just a proton. Because a hydrogen atom only has one electron and one proton, if the atom loses its electron it’s only a proton. It is this form of hydrogen that drives the enzyme ATP synthase in the mitochondria. The mitochondria is considered the “powerhouse” of the cell as it produces the majority of the ATP (adenosine triphosphate), which is the energy currency of our cells. The hydrogen ion (H+) is what is responsible for the pH of the water (i.e. acidic or alkaline). Water dissociates to form protons (H+) and hydroxides (OH–). That is: H2O => H+ + OH–. This is called the “self-ionization” of water.
pH is the –log of the hydrogen ion (H+) concentration. So, the more H+ ions the lower the pH, or the more acidic the solution is. (click here for more information on pH and see why H+ ions don’t really exist, and what pH really is.)